What Is The Hybridization Of Carbon . The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. These orbitals then bond with four hydrogen. with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. the sp 3 (pronunciation: a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat trigonal or. a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways.
from www.numerade.com
a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat trigonal or. the sp 3 (pronunciation: with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen. The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond.
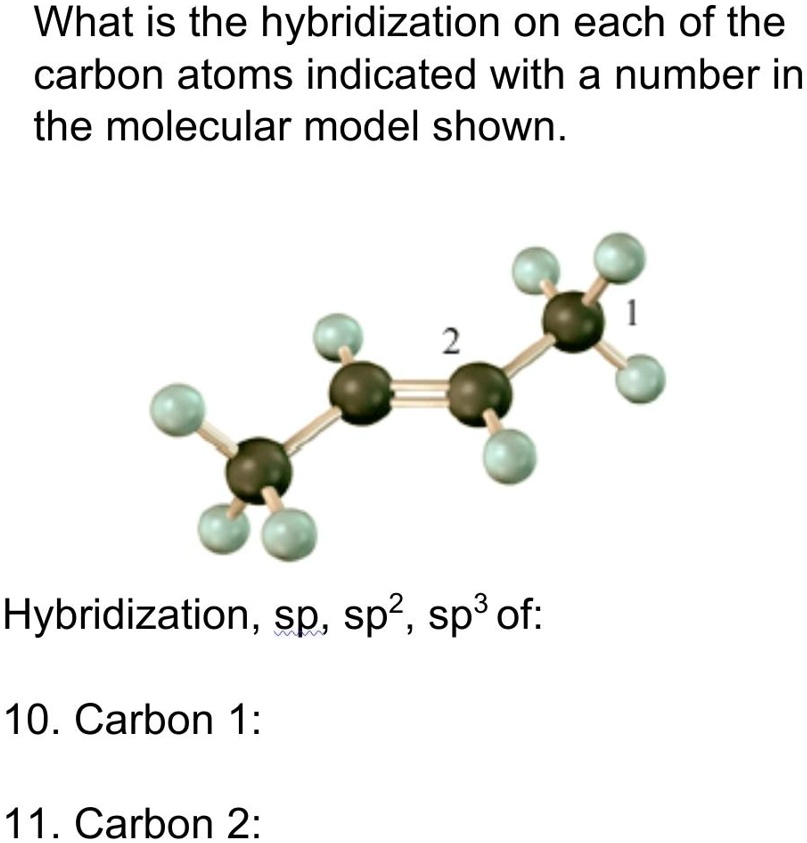
SOLVEDWhat is the hybridization on each of the carbon atoms indicated
What Is The Hybridization Of Carbon the sp 3 (pronunciation: The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. These orbitals then bond with four hydrogen. the sp 3 (pronunciation: when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat trigonal or.
From www.studyxapp.com
1 draw and determine the hybridization of the carbon atom in a What Is The Hybridization Of Carbon when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. The bond formed by the sp. What Is The Hybridization Of Carbon.
From www.numerade.com
SOLVEDa. What is the hybridization of each of the carbon atoms in the What Is The Hybridization Of Carbon when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. These orbitals then bond with four hydrogen. a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat. What Is The Hybridization Of Carbon.
From www.researchgate.net
a Atomic Chybridization. b C hybridization in carbonbased materials What Is The Hybridization Of Carbon with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the. What Is The Hybridization Of Carbon.
From www.youtube.com
What is the type of hybridisation of carbon atoms marked with star What Is The Hybridization Of Carbon the sp 3 (pronunciation: These orbitals then bond with four hydrogen. with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. when two sp 2 hybridized carbon. What Is The Hybridization Of Carbon.
From www.researchgate.net
1 The three hybridized form of carbon sp hybridization occurs only in What Is The Hybridization Of Carbon carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other. What Is The Hybridization Of Carbon.
From chem.libretexts.org
Hybridization Chemistry LibreTexts What Is The Hybridization Of Carbon The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the. What Is The Hybridization Of Carbon.
From www.windward.solutions
P4s3 hybridization What Is The Hybridization Of Carbon These orbitals then bond with four hydrogen. with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. the sp 3 (pronunciation: The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. when two sp 2 hybridized carbon. What Is The Hybridization Of Carbon.
From www.chegg.com
Solved What is the hybridization of carbon in C2O42 ? What Is The Hybridization Of Carbon The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen. a carbon atom bound to three atoms (two single bonds,. What Is The Hybridization Of Carbon.
From www.vrogue.co
Identify The Hybridization Of The Carbons In Each Mol vrogue.co What Is The Hybridization Of Carbon with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. The bond formed by the sp 2 orbitals is a sigma bond, and. What Is The Hybridization Of Carbon.
From quizlet.com
What is the hybridization of the carbon atom in carbon dioxi Quizlet What Is The Hybridization Of Carbon a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat trigonal or. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. carbon's 2s and all. What Is The Hybridization Of Carbon.
From chem.libretexts.org
Hybridization Chemistry LibreTexts What Is The Hybridization Of Carbon carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat trigonal or. with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. when two. What Is The Hybridization Of Carbon.
From www.doubtnut.com
What is the state of hybridization of carbon in butane? What Is The Hybridization Of Carbon These orbitals then bond with four hydrogen. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a. What Is The Hybridization Of Carbon.
From www.slideshare.net
Hybridisation of Carbon Atomic Orbitals What Is The Hybridization Of Carbon the sp 3 (pronunciation: The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat trigonal or. when two sp 2 hybridized. What Is The Hybridization Of Carbon.
From quizlet.com
What is the hybridization of each carbon atom in acetonitril Quizlet What Is The Hybridization Of Carbon when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen. a carbon atom bound. What Is The Hybridization Of Carbon.
From www.slideshare.net
Hybridisation of Carbon Atomic Orbitals What Is The Hybridization Of Carbon the sp 3 (pronunciation: carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. a carbon atom bound to three. What Is The Hybridization Of Carbon.
From avantecnica.qualitypoolsboulder.com
Hybridization Definition, Types, Rules, Examples What Is The Hybridization Of Carbon a carbon atom bound to three atoms (two single bonds, one double bond) is sp 2 hybridized and forms a flat trigonal or. These orbitals then bond with four hydrogen. with sp hybridization, each carbon has two sp hybrid orbitals and two unhybridized 2p orbitals. the sp 3 (pronunciation: The bond formed by the sp 2 orbitals. What Is The Hybridization Of Carbon.
From chem.libretexts.org
5.4 Hybridization of Carbon Chemistry LibreTexts What Is The Hybridization Of Carbon The bond formed by the sp 2 orbitals is a sigma bond, and the bond formed by the p orbitals is called a pi bond. the sp 3 (pronunciation: These orbitals then bond with four hydrogen. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head,. What Is The Hybridization Of Carbon.
From frank-khodges.blogspot.com
Describe the Orbitals Used by the Carbon Atom in Bonding What Is The Hybridization Of Carbon a molecule of methane, ch 4, consists of a carbon atom surrounded by four hydrogen atoms at the corners of a tetrahedron. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head, and two p orbitals approach each other sideways. These orbitals then bond with four. What Is The Hybridization Of Carbon.